Compound Information
|
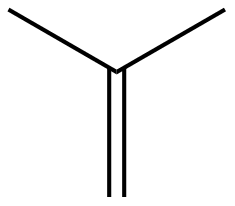
|
Property Availability
For this compound, WTT contains critically evaluated recommendations for:
(Please note that if more than 50 points are used for
regression, only the 50 most-constraining points are reported)
- Triple point temperature (Crystal 1, Liquid, and Gas)
5 experimental data points - Normal boiling temperature (Liquid and Gas)
- Critical temperature (Liquid and Gas)
5 experimental data points - Critical pressure (Liquid and Gas)
4 experimental data points - Boiling temperature (Liquid in equilibrium with Gas) as a function of Pressure
Pressure from 0.000677517 kPa to 4009.8 kPa - Phase boundary pressure (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 418.09 K
49 experimental data points - Critical density (Liquid and Gas)
3 experimental data points - Density
- Density (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 418.09 K
50 experimental data points - Density (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa
50 experimental data points - Density (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa
17 experimental data points - Density (Gas in equilibrium with Liquid) as a function of Temperature
Temperature from 132.4 K to 418.09 K
3 experimental data points
- Density (Liquid in equilibrium with Gas) as a function of Temperature
- Isobaric coefficient of expansion
- Isobaric coefficient of expansion (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa - Isobaric coefficient of expansion (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa
- Isobaric coefficient of expansion (Liquid) as a function of Temperature and Pressure
- Isothermal compressibility
- Isothermal compressibility (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa - Isothermal compressibility (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa
- Isothermal compressibility (Liquid) as a function of Temperature and Pressure
- Thermal pressure coefficient
- Thermal pressure coefficient (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa - Thermal pressure coefficient (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa
- Thermal pressure coefficient (Liquid) as a function of Temperature and Pressure
- 2nd virial coefficient (Gas) as a function of Temperature
Temperature from 132.4 K to 550 K
4 experimental data points - 3rd virial coefficient (Gas) as a function of Temperature
Temperature from 132.4 K to 550 K - Enthalpy of phase transition (Crystal 1 to Liquid in equilibrium with Gas)
1 experimental data points - Enthalpy of vaporization or sublimation (Liquid to Gas) as a function of Temperature
Temperature from 132.4 K to 418.09 K
1 experimental data points - Heat capacity at saturation pressure (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 417.9 K
21 experimental data points - Heat capacity at constant pressure
- Heat capacity at constant pressure (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa - Heat capacity at constant pressure (Ideal Gas) as a function of Temperature
Temperature from 50 K to 3000 K - Heat capacity at constant pressure (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa
- Heat capacity at constant pressure (Gas) as a function of Temperature and Pressure
- Enthalpy
- Enthalpy (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.41 K to 409.605 K - Enthalpy (Ideal Gas) as a function of Temperature
Temperature from 50 K to 3000 K
- Enthalpy (Liquid in equilibrium with Gas) as a function of Temperature
- Entropy
- Entropy (Ideal Gas) as a function of Temperature and Pressure
Temperature from 50 K to 3000 K - Entropy (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 418.09 K
- Entropy (Ideal Gas) as a function of Temperature and Pressure
- Adiabatic compressibility
- Adiabatic compressibility (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa - Adiabatic compressibility (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa
- Adiabatic compressibility (Liquid) as a function of Temperature and Pressure
- Pressure coefficient of enthalpy (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa - Joule-Thomson coefficient (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa - Speed of sound
- Speed of sound (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 418.09 K - Speed of sound (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.09 K
Pressure from 0.05 kPa to 50000 kPa - Speed of sound (Gas in equilibrium with Liquid) as a function of Temperature
Temperature from 132.4 K to 418.09 K - Speed of sound (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa
- Speed of sound (Liquid in equilibrium with Gas) as a function of Temperature
- Refractive index (Liquid) as a function of Wavelength, Temperature, and Pressure
Temperature from 213.046 K to 264.007 K
19 experimental data points - Surface tension (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 418.09 K
15 experimental data points - Viscosity
- Viscosity (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa
7 experimental data points - Viscosity (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.089 K
Pressure from 0.05 kPa to 50000 kPa - Viscosity (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 417.9 K
- Viscosity (Gas) as a function of Temperature and Pressure
- Thermal conductivity
- Thermal conductivity (Gas) as a function of Temperature and Pressure
Temperature from 132.4 K to 550 K
Pressure from 0.05 kPa to 4020 kPa - Thermal conductivity (Liquid in equilibrium with Gas) as a function of Temperature
Temperature from 132.4 K to 417.9 K - Thermal conductivity (Liquid) as a function of Temperature and Pressure
Temperature from 132.4 K to 418.089 K
Pressure from 0.05 kPa to 50000 kPa
- Thermal conductivity (Gas) as a function of Temperature and Pressure
- Enthalpy of formation
- Enthalpy of formation (Gas)
1 experimental data points - Enthalpy of formation (Liquid)
- Enthalpy of formation (Gas)
About WTT
NIST/TRC Web Thermo Tables (WTT)
NIST Standard Reference Subscription Database 3 - Professional Edition
Version 2-2012-1-Pro
This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics. These data were generated through dynamic data analysis, as implemented in the NIST ThermoData Engine software package [1, 2, 3, 4, 5, 6]. Some critically evaluated data from the historical TRC Thermodynamic Tables archive [7, 8] are included, also. As of May 2012, the Professional Edition contains information on 28432 compounds and total of 531486 evaluated data points. The properties covered by both versions (32 total) are described in Properties and Implemented Models.
Developed by Kenneth Kroenlein, Chris D. Muzny, Andrei F. Kazakov, Vladimir Diky, Robert D. Chirico, Joseph W. Magee, Ilmutdin Abdulagatov and Michael Frenkel.
Thermodynamics Research Center (TRC)
Thermophysical Properties Division
National Institute of Standards and Technology
Boulder CO 80305-3337
Questions and comments should be addressed to Dr. Michael Frenkel .
DISCLAIMER: The National Institute of Standards and Technology (NIST) uses its best efforts to deliver a high-quality copy of the database and to verify that the methods and data contained therein have been selected on the basis of sound scientific judgement. However, NIST makes no warranties to that effect, and NIST shall not be liable for any damage that may result from errors or omissions in the program and database.
Distributed by:
Standard Reference Data Program
National Institute of Standards and Technology
Gaithersburg MD 20899
©2012 copyright by the US Secretary of Commerce on
behalf of the United States of America. All rights reserved.
Privacy Policy/Security Notice/Accessibility Statement/Disclaimer/Freedom of Information Act (FOIA)
The TRC Group is part of the Thermophysical Properties Division in NIST's Material Measurement Laboratory
The National Institute of Standards and Technology is an agency of the U.S. Department of Commerce